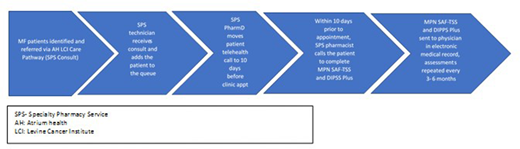
Background:
MF patients (pts) can have significant disease-associated symptoms. Guidelines recommend evaluation of pts' symptom burdens and prognostic risk scores at clinic visits. The myeloproliferative neoplasm symptom assessment form total symptom score (MPN-SAF TSS) is a standardized system that evaluates 10 specific MPN-related symptoms on a scale of 1-10. Furthermore, the dynamic prognostic scoring system plus (DIPSS plus) has been validated as a risk stratification tool for MF pts at any point during the disease course. At our institution, leukemia physicians have created an MF pathway to guide practitioners throughout a large healthcare system with many locations. The pathway recommends MPN-SAF TSS and DIPSS plus risk score with clinic visits occurring every 3-6 months. Unfortunately, the traditional approach to obtaining the MPN-SAF TSS and DIPSS plus score is burdensome for many providers and pts. Moreover, a recent analysis (Verstovsek Ann Hematol 2020) demonstrates that risk prognostication is very often performed incorrectly. We designed a study to streamline workflow by incorporating our specialty pharmacy to assist in the completion of the MPN-SAF TSS and DIPSS plus score via telephone prior to clinic visits. We hypothesized that partnership between our physicians and the specialty pharmacy may benefit pts by increasing adherence to the MF pathway.
Methods:
This study is conducted in two parts, a retrospective and a prospective phase. A review 12 months prior to start of study was completed to gain historical insight on provider adherence to the MF pathway, with attention to MPN-SAF TSS and DIPSS plus. Then, a prospective study was designed to include new or established MF pts. Pts would be followed for 12 months after start of study. The pilot study was open only at our largest site, home to a subspecialty leukemia clinic where disease-specific physicians practice.
For pts who enroll in the prospective study, specialty pharmacists complete both the MPN-SAF TSS and the DIPSS plus score ~3 months via phone prior to clinic visits. Calls for each pt are completed within 10 days of the pt's next visit. If the pharmacist is unable to reach the pt, the physician is notified via EMR message. Completed assessments are documented in the EMR. All enrolled pts are evaluated for MF pathway adherence during the study period.
Results:
We reviewed system-wide charts for 12 months prior to March 1, 2020 to assess if DIPSS and/or MPN SAF forms were documented for MF patients. 79 MF pts, treated by 32 physicians, were identified. Patients in remission after allogeneic stem cell transplantation were excluded. 36 pts (46%) had the MPN-SAF TSS completed and 53 pts (67%) had DIPSS plus performed at least once in the preceding 12 months. 45 pts, treated by 8 physicians, were followed primarily at the pilot site. 27 pts (60%) had MPN-SAF TSS documented and 38 (84%) had DIPSS Plus performed at least once in the preceding 12 months. The prospective study was initiated on March 1, 2020. As of July 15, 2020, 32 patients have been screened for enrollment by treating physicians. This includes 3 newly diagnosed patients not included in the retrospective analysis. 22 pts have had their initial assessments completed. 2 pts could not be reached, 1 of which died prior to the next clinic visit, 2 pts declined the study, and 6 pts had not yet had clinic visits. Of the 22 pts consented and contacted for the study, 100% had the MPN-SAF TSS and DIPSS Plus score documented with their first visit during the study period. To date 11 pts (of the initial 22) have undergone reevaluation; 100% had the MPN-SAF TSS and DIPSS Plus score documented with their 3-month follow-up visit.
Conclusion:
Many practicing clinicians perform MPN-SAF TSS and DIPSS plus scores for MF pts. However, there are lapses in the uptake of both. At our institution, the DIPSS plus score is performed more commonly than the MPN-SAF TSS. This early analysis suggests that a telemedicine approach and partnership between specialty pharmacy and physicians can optimize clinical workflow. With the initial success of this pilot, our program is expanding study enrollment to include regional clinics. Due to the COVID19 pandemic, there is now heightened interest in telemedicine practices. Preliminary data from this study suggest that a multidisciplinary approach incorporating telemedicine for MF pts provides an effective approach to measuring patient symptom burdens and assigning prognostic categories.
Chojecki:Novartis: Other: Investigator Meeting Attendance; Incyte: Research Funding. Shah:Incyte: Speakers Bureau; Pharmacyclics: Speakers Bureau. Knight:Foundation for Financial Planning: Research Funding. Ai:Celgene: Speakers Bureau; Incyte: Speakers Bureau. Avalos:Best Practice-Br Med J: Patents & Royalties: receives royalties from a coauthored article on evaluation of neutropenia; Juno: Membership on an entity's Board of Directors or advisory committees. Copelan:Amgen: Membership on an entity's Board of Directors or advisory committees. Grunwald:Janssen: Research Funding; Genentech/Roche: Research Funding; Abbvie: Consultancy; Daiichi Sankyo: Consultancy; Abbvie: Consultancy; Abbvie: Consultancy; Trovagene: Consultancy; Incyte: Consultancy, Research Funding; Celgene: Consultancy; Incyte: Consultancy, Research Funding; Agios: Consultancy; Janssen: Research Funding; Astellas: Consultancy; Daiichi Sankyo: Consultancy; Trovagene: Consultancy; Premier: Consultancy; Merck: Consultancy; Genentech/Roche: Research Funding; Forma Therapeutics: Research Funding; Agios: Consultancy; Celgene: Consultancy; Celgene: Consultancy; Cardinal Health: Consultancy; Pfizer: Consultancy; Trovagene: Consultancy; Premier: Consultancy; Astellas: Consultancy; Premier: Consultancy; Merck: Research Funding; Astellas: Consultancy; Merck: Consultancy; Genentech/Roche: Research Funding; Daiichi Sankyo: Consultancy; Agios: Consultancy; Amgen: Consultancy; Amgen: Consultancy; Cardinal Health: Consultancy; Merck: Consultancy; Cardinal Health: Consultancy; Pfizer: Consultancy; Amgen: Consultancy; Pfizer: Consultancy; Incyte: Consultancy, Research Funding; Forma Therapeutics: Research Funding; Forma Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.